Purchasing over $3,500? Contact us for further discounts. Over 250,000+ Products Available and Growing!
AAAAllied HealthcareAmvexAnesthesia AssociatesArrow InternationalAspen Surgical/NeedlesBaxaorationBayerB&BBeckman CoulterBerry PlasticsBionixCandela/LUNARClinical LaboratoryCliniqaCommon SenseImplantsIncontinenceIndicators and SignageInstrumentsI.V. TherapyKits, Packs & TraysLabelsMannequins and ModelsMasksMetal/Plastic/PaperMicroaire Surgical InstrumentsNeedles and SyringesNurse Assist
AAAAllied HealthcareAmvexAnesthesia AssociatesArrow InternationalAspen Surgical/NeedlesBaxaorationBayerB&BBeckman CoulterBerry PlasticsBionixCandela/LUNARClinical LaboratoryCliniqaCommon SenseConMed LinvatecCorneal ShieldCoretex
DeRoyalDevilbissDonovan IndustriesDraegerDynarexEndorphinExel InternationalFabrication EnterprisesFisher Scientific
MedlineMedtec MedicalMedtronics-SurgicalMedtronics - XomedMoore MedicalNascoNeotech Products, INC.New World ImportsNor-Lake ScientificNorth Coast MedicalNu-Hope LaboratoriesOhio Medical
Pall Life SciencesPatterson MedicalPhilips HealthcareR&B Wire Products:Industrial Laundry & Linen Transportation EquipmentRemington MedicalRespironics


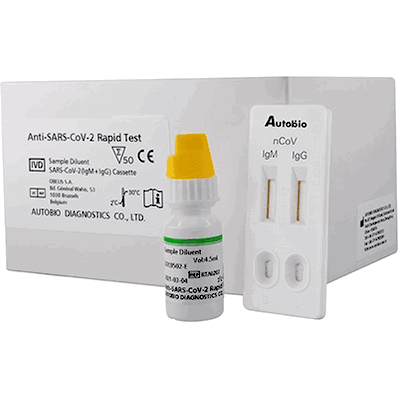
Anti-@sarom S-Cov-2 Rapid Test, COVID-19, 50 tests per kit
THIS IS ONLY SOLD TO DR's
INDIVIDUALS CANNOT ORDER
This test is for use by licensed laboratories and does not apply to at-home testing.
Item#: Aut-RTA0203-KT
$739.70
Anti-@sarom S-Cov-2 Rapid Test, COVID-19, 50 tests per kit, by AutoBio Diagnostics
DISCLOSURE
Hardy Diagnostics has permission to sell this test under the FDA’s Emergency Use Authorization (EUA) program , which is currently under review.
Please note the following information:
This test has not been reviewed by FDA Negative results do not rule out SARS-CoV-2 infection, particularly in those who have been in recent contact with the virus, due to the lag time between exposure and the patient’s antibody response. Follow-up testing with a molecular diagnostic test should be considered in order to rule out infection in these individuals.
Results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection or to inform of infection status.
Positive results may be due to past or present infections with non-SARS-CoV-2 coronavirus strains, such as coronavirus HKU1, NL63, OC43 or 229E.
This test is for use by licensed laboratories and does not apply to at-home testing.
Not to be used for blood donor screening
DISCLOSURE
Hardy Diagnostics has permission to sell this test under the FDA’s Emergency Use Authorization (EUA) program , which is currently under review.
Please note the following information:
This test has not been reviewed by FDA Negative results do not rule out SARS-CoV-2 infection, particularly in those who have been in recent contact with the virus, due to the lag time between exposure and the patient’s antibody response. Follow-up testing with a molecular diagnostic test should be considered in order to rule out infection in these individuals.
Results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection or to inform of infection status.
Positive results may be due to past or present infections with non-SARS-CoV-2 coronavirus strains, such as coronavirus HKU1, NL63, OC43 or 229E.
This test is for use by licensed laboratories and does not apply to at-home testing.
Not to be used for blood donor screening
- PRODUCT DETAILS
- GOT QUESTIONS
- RECENTLY VIEWED
Price shown is sold as per Pack
| Manufacturer # | RTA0203 |
| Manufacturer | AutoBio Diagnostics |
| Application | Anti-SARS-Cov-2 Rapid Test |
Features
- Results in 15 minutes
- No special equipment or facilities needed.
- Easy-to-interpret results.
- Built-in-controls
- Room temperature storage
- Sensitivity - 97.4%
- Specificity - 96.2%
- Intended for the presumptive detection of IgG and IgM antibodies from the SARS-CoV-2 virus in plasma, serum, or capillary finger prick blood from individuals with signs and symptoms of COVID-19 infection. PK 50 (50 tests per kit)
- One of the world’s first rapid lateral flow immunoassays for the diagnosis of COVID-19 disease. The test has been used widely by the Chinese CDC to combat infections and is now available globally. This kit is also CE Marked-IVD. This test detects both early marker (IgM) and late marker (IgG) antibodies in human finger-prick (capillary), serum, and plasma samples, from anticoagulated blood.
- The test can be used for rapid screening of carriers of the virus that are symptomatic or asymptomatic. Recent studies suggest that a high percentage of patients show no clinical symptoms of the virus, thus screening patients is vitally important. The test is ideally suited for hospitals and testing laboratories that perform highly complex testing.
- The Anti-SARS-Cov-2 Rapid Test can be used to screen patients suspected of having been infected by the novel coronavirus. However, results of test should not be the only basis for diagnosis. Results should be used in combination with clinical observations and other testing methods such as nucleic acid PCR test.
- The BEST WAY to contact is by EMAIL - Use our "Contact Us" page, if you need immediate response, write URGENT on the subject line.
- Emails are monitored 9 am - 4 pm, Mon-Fri, except Holidays, California Time.
- Can't find the Exact Size? Contact us ( use "contact us" page) and we will get you the info.
- Need it Yesterday? we have options for 2-3 days, 4-5 days use the check out page shipping options. Take note: the clock begins the day after the order and excludes week-ends and Holidays.
- Can't Find the Exact Item but you found the Manufacturer? Send us the Info, and we will locate it for you.
- Need a Quote? Please email us, be accurate with part#, description and quantities.
- Overseas Customers? Contact us first before making your purchase.