Purchasing over $3,500? Contact us for further discounts. Over 250,000+ Products Available and Growing!
AAAAllied HealthcareAmvexAnesthesia AssociatesArrow InternationalAspen Surgical/NeedlesBaxaorationBayerB&BBeckman CoulterBerry PlasticsBionixCandela/LUNARClinical LaboratoryCliniqaCommon SenseImplantsIncontinenceIndicators and SignageInstrumentsI.V. TherapyKits, Packs & TraysLabelsMannequins and ModelsMasksMetal/Plastic/PaperMicroaire Surgical InstrumentsNeedles and SyringesNurse Assist
AAAAllied HealthcareAmvexAnesthesia AssociatesArrow InternationalAspen Surgical/NeedlesBaxaorationBayerB&BBeckman CoulterBerry PlasticsBionixCandela/LUNARClinical LaboratoryCliniqaCommon SenseConMed LinvatecCorneal ShieldCoretex
DeRoyalDevilbissDonovan IndustriesDraegerDynarexEndorphinExel InternationalFabrication EnterprisesFisher Scientific
MedlineMedtec MedicalMedtronics-SurgicalMedtronics - XomedMoore MedicalNascoNeotech Products, INC.New World ImportsNor-Lake ScientificNorth Coast MedicalNu-Hope LaboratoriesOhio Medical
Pall Life SciencesPatterson MedicalPhilips HealthcareR&B Wire Products:Industrial Laundry & Linen Transportation EquipmentRemington MedicalRespironics


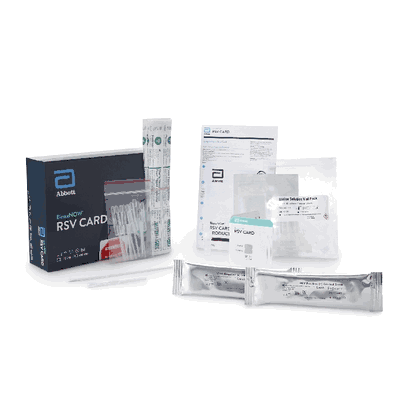
Respiratory Test Kit, Respiratory Syncytial Virus Test (RSV) Infectious Disease Immunoassay Rapid Test Card Format, sold as 10 Tests/Box by BinaxNOW, Abbott Rapid Dx 430100
Contents Include:
(10) Test Cards, Transfer Pipettes, Positive Control Swab (Inactivated RSV), Negative Control Swab, Elution Solution and NP Patient Swabs (with CLIA Waived Kits), Product Insert, Procedural Card
We can only ship to Businesses and Clinics.
Item#: Inv-430-100-BX
$244.22
- PRODUCT DETAILS
- GOT QUESTIONS
- RECENTLY VIEWED
| Dealer # | 533820 |
| Manufacturer # | 430100 |
| Brand | BinaxNOW® |
| Manufacturer | Abbott Rapid Dx North America LLC |
| Country of Origin | United States |
| Application | Respiratory Test Kit |
| CLIA Classification | CLIA Waived |
| CLIA Classified | CLIA Waived |
| Contents 1 | (10) Test Cards, Transfer Pipettes, Positive Control Swab (Inactivated RSV), Negative Control Swab, Elution Solution and NP Patient Swabs (with CLIA Waived Kits), Product Insert, Procedural Card |
| Number of Tests | 10 Tests |
| Reading Type | Visual Read |
| Sample Type | Nasopharyngeal Swab / Nasal Wash Sample |
| Specialty | Immunoassay |
| Test Format | Test Card Format |
| Test Kit Type | Rapid |
| Test Method | Lateral Flow Method |
| Test Name | Respiratory Syncytial Virus Test (RSV) |
| Test Type | Infectious Disease Immunoassay |
| Time to Results | 15 Minute Results |
| UNSPSC Code | 41116126 |
Features
- BinaxNOW® RSV Card is a rapid immunochromatographic assay for the qualitative detection of respiratory syncytial virus (RSV) fusion protein antigen in nasal wash and nasopharyngeal swab specimens from symptomatic patients
- Shelf Life: 24 months from the date of manufacture
- Retrospective nasal wash Sensitivity: 89% Specificity: 100%
- Prospective NP swab Sensitivity: 93% Specificity 93%
- This test is intended for in vitro diagnostic use to aid in the diagnosis of respiratory syncytial virus infections in neonatal and pediatric patients under the age of 5
- The BEST WAY to contact is by EMAIL - Use our "Contact Us" page, if you need immediate response, write URGENT on the subject line.
- Emails are monitored 9 am - 4 pm, Mon-Fri, except Holidays, California Time.
- Can't find the Exact Size? Contact us ( use "contact us" page) and we will get you the info.
- Need it Yesterday? we have options for 2-3 days, 4-5 days use the check out page shipping options. Take note: the clock begins the day after the order and excludes week-ends and Holidays.
- Can't Find the Exact Item but you found the Manufacturer? Send us the Info, and we will locate it for you.
- Need a Quote? Please email us, be accurate with part#, description and quantities.
- Overseas Customers? Contact us first before making your purchase.