Cranial Access Kits By Integra Lifesciences -
We only sell to businesses, clinics, government facilities, individuals may contact us by email - info@go3a.com
- PRODUCT DETAILS
- GOT QUESTIONS
- RECENTLY VIEWED
| Unit of Measure | Conversion | Net / Gross Weight (lbs) | Volume (cubic ft) | Dimensions (inch) L x W x H | GTIN |
|---|---|---|---|---|---|
| Each (EA) | 1.0 Each | 0.0 / 1.85 | 0.347 | 0.0 x 0.0 x 0.0 | |
| Case (CS) | 5.0 Each | 0.0 / 9.25 | 1.736 | 20.0 x 15.0 x 10.0 |
| Packaging | 5 Each / Case |
| Manuf / Supplier | Integra Lifesciences Corp |
| Manuf / Supplier # | INS5HND |
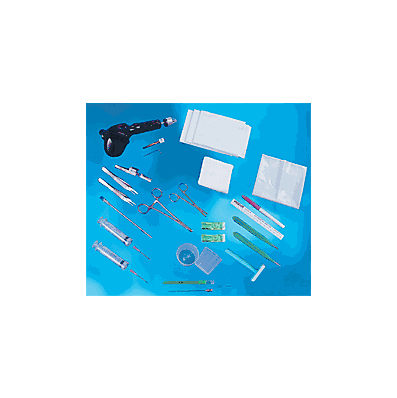
This Kit Contains
- Razor
- 12cc Safety Syringes (2)
- Cold applications
- 18G x 1- 1/2" Needles (2)
- 25G x 5/8" Needle
- 18G x 3-1/2" Spinal Needle
- 12G x 5 1/2" Ventricular Needle
- 15" x 15" Fenestrated Drape with Barrier
- 18" x 26" White, Absorbent Towels (3)
- 4" x 4" Gauze Sponges (10)
- Hand Drill
- #11 Scalpel with Handle
- #15 scalpel with Handle
- Adson Forceps, serrated
- Adson Forceps, 1 x 2 teeth
- Needle Holder
- 2 oz. Medicine Cup (2)
- Self-Retaining Retractor
- Scissors
- 5.31mm Drill Bit Assembly
- 3-0 Nylon Suture (packaged outside kit)
- Marker
- Flexible Ruler
- Hex Wrench
Indications:
The Integra Cranial Access Kit allows for access to the subarachnoid space or the lateral ventricles of the brain. The kit is intended to be used with an external drainage and monitoring system in selected patients to reduce intracranial pressure (ICP), to monitor CSF, to provide temporary drainage of CSF, and to monitor ICP.
Contraindications:
This product is not designed, sold, or intended for use except as indicated.
Warnings:
Patients undergoing external CSF drainage and ICP monitoring must be kept under close supervision with trained personnel familiar with the use of monitoring techniques. All personnel should be familiar with the information provided in this booklet. Extreme care should be taken during the preparation and performance of the drilling procedure to maintain sterile technique. The hand drill with variable chuck and drill bit assembly must be used properly to prevent penetration or damage to the dura.
Precautions:
Inform the patient or his/her representatives of possible complications associated with use of this system. Cranial Access components should avoid contact with lint, glove talc, and other surface contaminants. Contents of the kit are sterile if the package is unopened and undamaged. Use aseptic technique throughout this procedure. The drill, with drill bit, adjustable chuck and adjustable stop must me used properly in order to prevent drilling too deeply and causing injury or damage to the dura, cortex and cortical vessels. Maintain insertion site with meticulous redressing according to physicians orders. Avoid over drainage of CSF during placement of ventricular catheters. The kit is designed for single use only. Do not re-sterilize. This product is intended for single use only. Reuse of the device can result in contamination and/or disease transmission. This product should not be re-sterilized. Re-sterilization may affect the performance characteristics and the safety of the device.
- The BEST WAY to contact is by EMAIL - Use our "Contact Us" page, if you need immediate response, write URGENT on the subject line.
- Emails are monitored 9 am - 4 pm, Mon-Fri, except Holidays, California Time.
- Can't find the Exact Size? Contact us ( use "contact us" page) and we will get you the info.
- Need it Yesterday? we have options for 2-3 days, 4-5 days use the check out page shipping options. Take note: the clock begins the day after the order and excludes week-ends and Holidays.
- Can't Find the Exact Item but you found the Manufacturer? Send us the Info, and we will locate it for you.
- Need a Quote? Please email us, be accurate with part#, description and quantities.
- Overseas Customers? Contact us first before making your purchase.