Purchasing over $3,500? Contact us for further discounts. Over 250,000+ Products Available and Growing!
AAAAllied HealthcareAmvexAnesthesia AssociatesArrow InternationalAspen Surgical/NeedlesBaxaorationBayerB&BBeckman CoulterBerry PlasticsBionixCandela/LUNARClinical LaboratoryCliniqaCommon SenseImplantsIncontinenceIndicators and SignageInstrumentsI.V. TherapyKits, Packs & TraysLabelsMannequins and ModelsMasksMetal/Plastic/PaperMicroaire Surgical InstrumentsNeedles and SyringesNurse Assist
AAAAllied HealthcareAmvexAnesthesia AssociatesArrow InternationalAspen Surgical/NeedlesBaxaorationBayerB&BBeckman CoulterBerry PlasticsBionixCandela/LUNARClinical LaboratoryCliniqaCommon SenseConMed LinvatecCorneal ShieldCoretex
DeRoyalDevilbissDonovan IndustriesDraegerDynarexEndorphinExel InternationalFabrication EnterprisesFisher Scientific
MedlineMedtec MedicalMedtronics-SurgicalMedtronics - XomedMoore MedicalNascoNeotech Products, INC.New World ImportsNor-Lake ScientificNorth Coast MedicalNu-Hope LaboratoriesOhio Medical
Pall Life SciencesPatterson MedicalPhilips HealthcareR&B Wire Products:Industrial Laundry & Linen Transportation EquipmentRemington MedicalRespironics


Swan-Ganz Pediatric Catheter, Oximetry withou
We only sell to businesses, clinics, government facilities, individuals may contact us by email - info@go3a.com
Item#: Edw-040F4-ea
$615.70
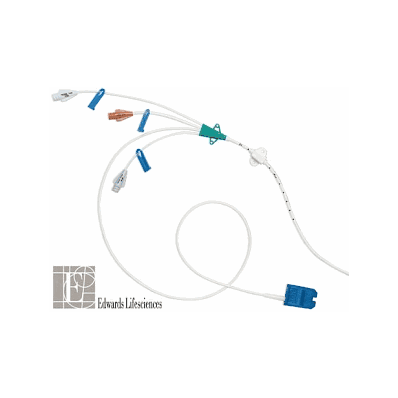
Swan-Ganz Pediatric Catheter, Oximetry without Thermistor (Each/1) - Edwards Lifescience
- PRODUCT DETAILS
- GOT QUESTIONS
- RECENTLY VIEWED
THESE ARE OVERSTOCK, LIMITED SUPPLIES. IF YOU ARE PLANNING TO PURCHASE CONTACT US FIRST BEFORE ORDERING.
Price shown is sold as EA/1
| Dealer # | AAA040F4 |
| Manufacturer # | 040F4 |
| Manufacturer | Edwards Lifescience |
| Application | Edwards Swan-Ganz Pediatric Catheter |
MORE DESCRIPTIONS
Determined to be “MR-safe” according to the classification method specified in the American Society for Testing and Materials (ASTM) International, Designation: F2503-08. Standard Practice for Marking Medical Devices and Other Items for Safety in the Magnetic resonance Environment. Made entirely from nonmetallic, non-conducting, and nonmagnetic materials. None of these devices have materials that are affected by the electromagnetic fields generated during an MRI procedure. Accordingly, these devices present no known hazard when used in or on patients who are undergoing MRI procedures.
Features
- Swan-Ganz Oximetery TD catheter enables monitoring of hemodynamic pressures, cardiac output, and continuous mixed venous oxygen saturation.
- Swan-Ganz VIP Oximetry TD catheter enables monitoring of hemodynamic pressures, cardiac output, and mixed venous oxygen saturation and provides an additional (VIP) lumen that allows for continuous infusion.
- Swan-Ganz Oximetry Paceport TD catheter is intended for use in patients who require hemodynamic monitoring when temporary transvenous pacing is anticipated.
- The BEST WAY to contact is by EMAIL - Use our "Contact Us" page, if you need immediate response, write URGENT on the subject line.
- Emails are monitored 9 am - 4 pm, Mon-Fri, except Holidays, California Time.
- Can't find the Exact Size? Contact us ( use "contact us" page) and we will get you the info.
- Need it Yesterday? we have options for 2-3 days, 4-5 days use the check out page shipping options. Take note: the clock begins the day after the order and excludes week-ends and Holidays.
- Can't Find the Exact Item but you found the Manufacturer? Send us the Info, and we will locate it for you.
- Need a Quote? Please email us, be accurate with part#, description and quantities.
- Overseas Customers? Contact us first before making your purchase.